Back to Precipitation Reactions Exercise 7. A hydrocarbon is a compound consisting mainly of carbon C and hydrogen H.

Precipitation Reaction A Reaction That Results In The Formation Of An Insoluble Product A Teaching Chemistry Chemistry Education Chemical Equations Chemistry
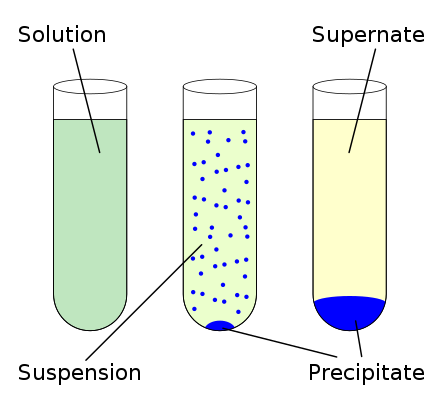
These insoluble salts formed in precipitation reactions are called precipitates.
. Precipitation reactions are best classified as which type of reaction2 points a. Precipitation reactions 1. The molecular equation is.
Precipitation reactions can be broad of three types. Ring test and flocculation tests. A precipitation reaction can occur when two solutions containing different salts are mixed and a cationanion pair in the resulting combined solution forms an insoluble salt.
This solid is referred to as a precipitate. What type of reaction is the generic equation A B AB. This salt then precipitates out of solution.
This is because much like rain falls from the sky the solid that forms will fall out of the solution to. Precipitation Reactions and Solubility Rules. Precipitation Reaction Definition and Meaning.
Precipitation Reactions and Solubility Rules. Precipitation reactions are best classified as which type of reaction. KOH aq HClO 4 aq K aq ClO 4.
Precipitation reactions acid-base reactions gas evolution reactions and oxidation-reduction reactions. Example of combination reaction. If a precipitation reaction does occur write a net ionic equation and identify the spectator ions.
Any time we have a compound breaking down into its elements or a compound forming smaller molecules this is a decomposition reaction. Whether or not it is a combination decomposition single replacement double replacement or combustion reaction. Determine if a precipitation reaction will occur if aqueous solutions of the substances in each pair are mixed.
A precipitation reaction is one in which dissolved substances react to form one or more solid products. Precipitation reaction can be broadly of three types. These reactions are common in.
The term precipitation reaction can be defined as a chemical reaction occurring in an aqueous solution where two ionic bonds combine resulting in the formation of an insoluble salt. We will only consider organic combustions where the fuel will always be a hydrocarbon and the oxidizer is oxygen. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement double replacement or metathesis reactions.
A reaction in which two or more reactants combine to form a single product is known as a combination reaction. We encounter combustion reactions often in our daily lives. Titanium can be obtained from its oxide by the reaction shown here.
2Na Cl 2 2NaCl. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement double replacement or metathesis reactions. What is the percent yield for.
May 15 2021 by Sagar Aryal. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement double replacement or metathesis reactions. Precipitation reactions are best classified as which type of reaction 2 points a from CHEMISTRY 181 at El Centro College.
Sometimes a reaction is a synthesis reaction and also a redox reaction. The liquid that remains when a precipitate forms is called the supernate. A KOH and HClO 4.
What type of reaction is the generic equation A BC AC B. How many moles of oxygen atoms are in sulfur tetraoxide. Synthesis decomposition single-displacement and double- displacement.
Precipitation reactions are best classified as which type of reaction. Combination reaction is also known as a synthesis reaction. Types of precipitation reaction.
When table salt Sodium Chloride is dissolved in water to form an aqueous solution the ions no longer bond together they separate into an array of positive and negative ions. A different model for classifying chemical reactions classifies reactions as. NaCl s Na aq Cl-aq 2.
Precipitation reactions are best classified as which type of reaction 2 points from CHEMISTRY 181 at El Centro College. Acid-base reactions are best classified as which type of reaction. A precipitation reaction is one in which dissolved substances react to form one or more solid products.
In a reaction in which one of the products has little to no solubility ability to dissolve in water a solid will form. July 27 2021. A precipitation reaction is one in which dissolved substances react to form one or more solid products.
It takes the form of X Y XY. Precipitation in agar with an electric field. A precipitation reaction is a type of chemical reaction in which two soluble salts in aqueous solution combine and one of the products is an insoluble salt called a precipitate.
Chemical reactions can be classified as. This type of reaction involves a reaction between a fuel and an oxidizer. Precipitation reactions in which a solid called a Which type of cloud produces precipitation.
This question asks you to identify based upon the descriptions of the chemical reactions. What must bind to the cross bridge for myosin to disconnect A bundle of muscle fibers is called a ____________ and is su. Circle which observation is not.
The dissolving of Sodium Chloride into water can be represented by the equation. When 420 g of TiO 2 react with 115 g C 935 g Ti are obtained. Precipitation refers to a chemical reaction that occurs in aqueous solution when two ions bond together to form an insoluble salt which is known as the precipitate.
Group of answer choices. If copper and bro. Precipitation reactions are usually double displacement.

Precipitation Chemistry Wikiwand

Chemistry Jokes And Puns With Explanations Chemistry Jokes Science Clipart Science Humor
Precipitation Reaction In Chemistry Precipitation Reaction Equation And Examples
0 Comments